LOW-GRADE SEROUS OVARIAN CANCER IS

Low-Grade Serous Ovarian Cancer (LGSOC) Clinical Trials
Clinical trials are happening right now for LGSOC
Did you know? The Food and Drug Administration (FDA) has not approved any treatment specifically for low-grade serous ovarian cancer (also known as LGSOC). But scientists are working hard to change that. There are different types of groundbreaking medicines being studied in people with LGSOC. In this section, we’ll tell you a little bit about the types of trials happening now, and how to learn more about them
Finding hope in clinical trials
Kat, a person with LGSOC, talks about her diagnosis, self-advocacy, and the importance of clinical trials.
Understanding clinical trials
You may have heard of clinical trials while researching LGSOC. Clinical trials are studies run by health care professionals (HCPs) that can determine if a new medicine or treatment works and is safe for people to use. All new medicines and medicine combinations must go through clinical trials before they can be approved by the FDA.
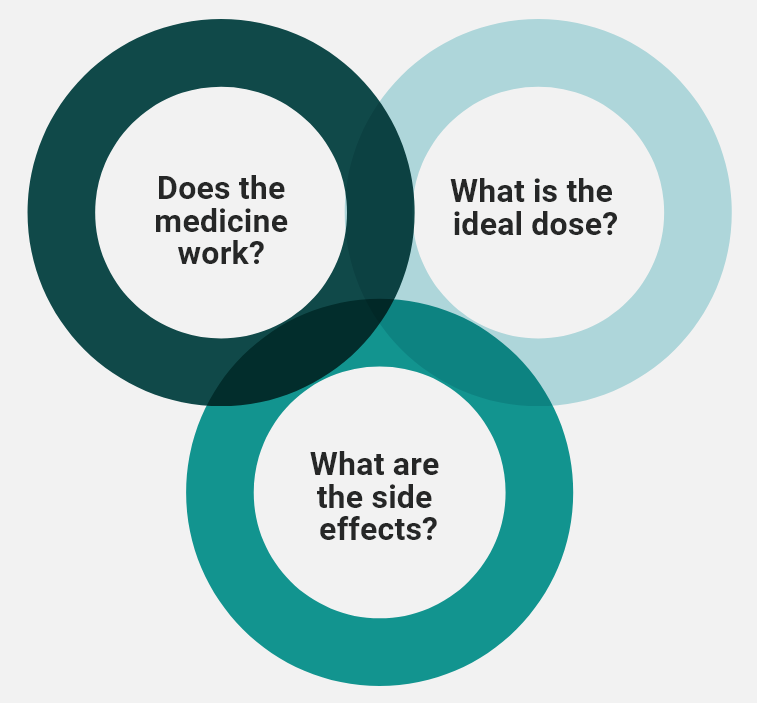
Clinical trials study different aspects of medicines and how they interact with the body. HCPs will determine the safest dose, which side effects are caused by the treatment, and if the treatment is effective.
Clinical trials for low-grade serous ovarian cancer
There are clinical trials going on right now that could lead to breakthrough treatments for people with LGSOC.
A variety of treatments are being studied. Treatments that have been shown to work in other cancers are being studied to see if they can also work in LGSOC. Groundbreaking new approaches that could lead to treating LGSOC in a completely different way are being tested as well.
By signing up for a clinical trial, you may have the chance to try one of these new medicines before they are approved by the FDA. Taking part in a clinical trial can be life-changing, so it’s important that you consider signing up for a clinical trial if you are eligible.
These studies are investigating treatments or outcomes that have not received approval from a health authority. There is no guarantee that the outcome of these studies will result in approval.
Patient Webinar: Let’s Talk About Clinical Trials
Hear from leading gynecologic oncologist, Dr. Jubilee Brown, on recent progress in research evaluating treatments for LGSOC. Dr. Brown also discusses the importance of clinical trials and resources for finding them.
How to find a clinical trial
Click on one of the links below and follow the instructions to find clinical trials for LGSOC. You can share the information you find with your doctor to start a conversation about clinical trials.
How to find a clinical trial
Click on one of the links below and follow the instructions to find clinical trials for LGSOC. You can share the information you find with your doctor to start a conversation about clinical trials.




