LGSOC Patient Impact Survey Results
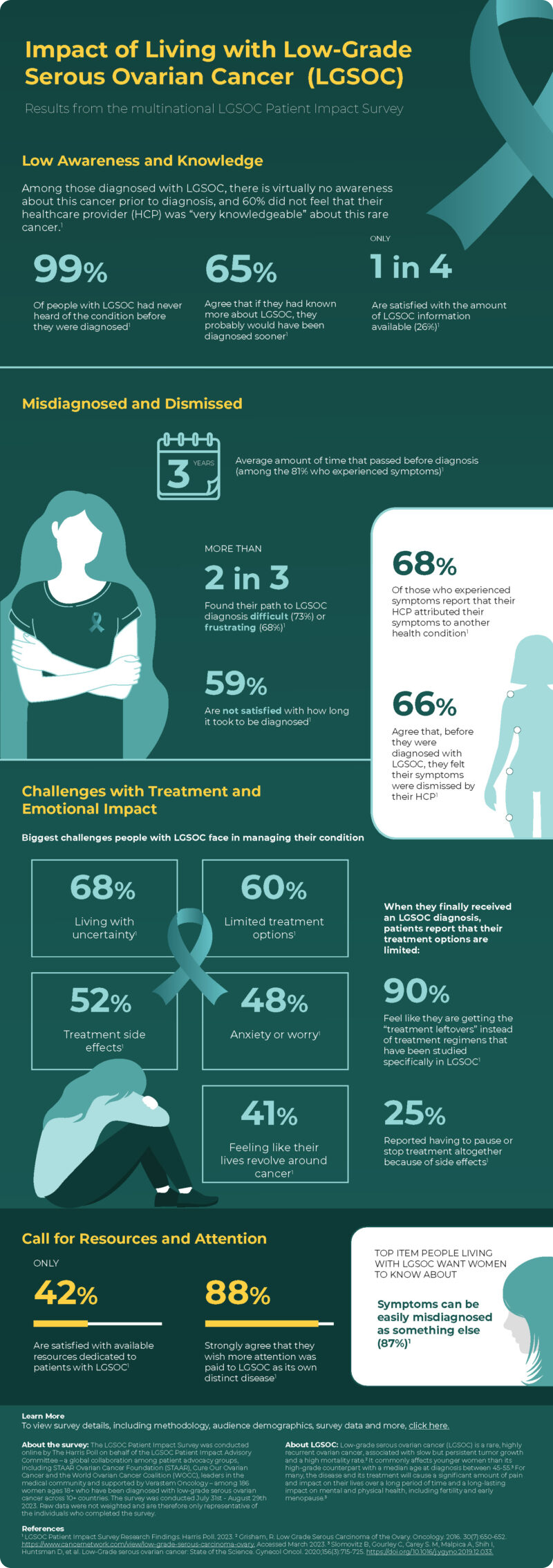
We are pleased to present the results from the LGSOC Patient Impact Survey, a first-of-its-kind, multi-national survey among women diagnosed with low-grade serous ovarian cancer (LGSOC). More than 180 participants were surveyed across 10 countries to assess patient knowledge, burden and impact of disease, and barriers to treatment.
LGSOC is a rare, highly recurrent ovarian cancer associated with slow but persistent tumor growth and a high mortality rate. This survey, administered by The Harris Poll and supported by Verastem Oncology, was conducted by leaders in the medical and advocacy communities to better understand and address the challenges of this rare and difficult to treat disease. Top global findings revealed:
- Among those surveyed who experienced disease symptoms (81%), it took an average of nearly three years to get an accurate LGSOC diagnosis
- Most report their path to diagnosis as difficult (75%) or frustrating (68%) – with many experiencing misdiagnosis (68%) or having their symptoms initially dismissed by a healthcare provider (66%)
- When it comes to educating others about LGSOC, 87% want women to know that symptoms can be easily misdiagnosed as another disease, and 82% agreed that it’s important to listen to your body and seek medical attention as soon as possible
To view survey details, including methodology, audience demographics, survey data and more, click the button below:
Hear from people with LGSOC
Learn more about the experiences people living with LGSOC share.
See also
LGSOC glossary
Use this glossary of commonly used words and phrases associated with LGSOC to help you feel more prepared when talking to your care team.